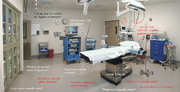
Device Use Environment: Environments for which the device is intended to be used
About[]
Device user interface includes all components of a device with which the user interacts, such as controls and displays (i.e., those parts of the device that users see, touch, and hear). The user interface also includes the device labeling, which includes package labels, any instructions for use in user manuals, package inserts, instructions on the device itself, and any accompanying informational materials.
To gain an understanding of the potential HFE/UE analyses that should be conducted for a particular device, you should consider:
Device use environment:
- Hospital, surgical suite, home, emergency use , public use, etc.
- Special environments (e.g., emergency transport, mass casualty event, sterile isolation, hospital intensive care unit)
- Interoperability ("can devices and users working nicely together?") with other devices.
The environments in which medical devices are used may present a range of complexities. Medical devices may be used under variable conditions involving such environmental attributes as space, lighting, noise levels, and activity. Examples of environmental hazards in the clinical setting include the following:
Rooms can be physically crowded or cluttered, making it difficult for people to maneuver in the space.
- The lighting level can be low, making it hard to see device displays or controls.
- The noise level can be high, making it hard to hear device operation feedback or audible alerts and alarms.
- The room can be busy with other people and activities, providing distractions that can confuse the device operator.
Non-clinical environments can present additional challenges. For example:
- Carpeting or stairs might make it hard to move medical devices around the space.
- The environment might not be clean.
- The utility service might not be reliable. In addition, the electrical outlets might not be grounded, and the water might not be clean.
- The temperature might be very high, which could cause devices to overheat (and make users’ hands sweaty), or the temperature might be very low, which could make devices inoperable (and make users’ fingers stiff and decrease sensitivity).
- The humidity might be very high, which could cause condensation to form, or very low, which could produce static electricity.
- Other individuals and activities in the vicinity may cause distractions.
- Unauthorized users, such as children, might be present and could hurt themselves (e.g., playing with a syringe), damage the device (e.g., chewing on or misconnecting the tubing), or change device settings (which might not be noticed by the operator before using the device the next time).
- Pets or vermin could contaminate or damage devices in the home.
- Electromagnetic interference from other equipment (e.g., cell phones and computer accessories) could affect medical device performance.
Use environments can also limit the effectiveness of visual and auditory displays (lighted indicators, auditory alarms and other signals) if they are not designed appropriately. For example, in noisy environments, the user might not be able to notice a device’s alarms if they are not sufficiently loud or distinctive. When multiple alarms occur for different devices or on the same device, or if alarms sound too often, i.e., “nuisance” alarms, the user could fail to notice them or be unable to make important distinctions among them. Similarly, motion and vibration can affect the degree to which people are able to perform fine physical manipulations such as typing on a keyboard or reading displayed information.[1]
Reference[]
- ↑ FDA. Draft Guidance for Industry and Food and Drug Administration Staff - Applying Human Factors and Usability Engineering to Optimize Medical Device Design. June 22, 2011. http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm259748.htm#2